Jeffrey A. Klein, MD, MPH,*† and Daniel R. Jeske, PhD†
BACKGROUND: Tumescent lidocaine anesthesia consists of subcutaneous injection of relatively large volumes (up to 4 L or more) of dilute lidocaine (≤1 g/L) and epinephrine (≤1 mg/L). Although tumescent lidocaine anesthesia is used for an increasing variety of surgical procedures, the maximum safe dosage is unknown. Our primary aim in this study was to measure serum lidocaine concentrations after subcutaneous administration of tumescent lidocaine with and without liposuction. Our hypotheses were that even with large doses (i.e., >30 mg/kg), serum lidocaine concentrations would be below levels associated with mild toxicity and that the concentration-time profile would be lower after liposuction than without liposuction.
METHODS: Volunteers participated in 1 to 2 infiltration studies without liposuction and then one study with tumescent liposuction totally by local anesthesia. Serum lidocaine concentrations were measured at 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, and 24 hours after each tumescent lidocaine infiltration. Area under the curve (AUC∞) of the serum lidocaine concentration-time profiles and peak serum lidocaine concentrations (Cmax) were determined with and without liposuction. For any given milligram per kilogram dosage, the probability that Cmax >6 μg/mL, the threshold for mild lidocaine toxicity was estimated using tolerance interval analysis.
RESULTS: In 41 tumescent infiltration procedures among 14 volunteer subjects, tumescent lidocaine dosages ranged from 19.2 to 52 mg/kg. Measured serum lidocaine concentrations were all <6 μg/mL over the 24-hour study period. AUC∞s with liposuction were significantly less than those without liposuction (P = 0.001). The estimated risk of lidocaine toxicity without liposuction at a dose of 28 mg/kg and with liposuction at a dose of 45 mg/kg was ≤1 per 2000.
CONCLUSIONS: Preliminary estimates for maximum safe dosages of tumescent lidocaine are 28 mg/kg without liposuction and 45 mg/kg with liposuction. As a result of delayed systemic absorption, these dosages yield serum lidocaine concentrations below levels associated with mild toxicity and are a nonsignificant risk of harm to patients. (Anesth Analg 2016;122:1350–9)
Tumescent lidocaine anesthesia (TLA) was developed for performing liposuction totally by local anesthesia with virtually no surgical blood loss.1,2 TLA has been extended to a wide range of other surgical procedures involving cutaneous, subcutaneous, breast, and vascular tissues.3–25 The maximum safe dosage of tumescent lidocaine for these procedures is unknown. There is a need for a pharmacokinetic-based estimate of the maximum safe milligram per kilogram dosage of tumescent lidocaine. 26,27
The package insert labeling approved by the United States Food and Drug Administration (FDA) for lidocaine with epinephrine states that the recommended maximal dosage is 7 mg/kg for infiltration local anesthesia. The FDA has no data to support this 7 mg/kg as the dosage limit, which was established in 1948 for epidural anesthesia. The liposuction guidelines of the American Society for Dermatologic Surgery recommended that the maximal safe milligram per kilogram dosage of tumescent lidocaine for liposuction totally by local anesthesia is 55 mg/kg.28
Tumescent lidocaine solution contains at most 1 g lido- caine and 1 mg epinephrine in 100 mL plus 10 mEq sodium bicarbonate in 10 mL added to 1000 mL of 0.9% physiologic saline for a final lidocaine concentration of 1 g per bag containing 1110 mL or 0.9 g/L (0.09%). Sodium bicarbonate reduces the stinging discomfort of large volume subcutaneous tumescent infiltration.29
Subcutaneous infiltration of large volumes of TLA solution causes the targeted tissue to become temporarily swollen and firm or tumescent. The resulting increased subcutaneous interstitial pressure spreads the TLA solution through adjacent tissues by bulk flow. Lidocaine toxicity is a function of serum lidocaine concentration. Dilute epinephrine produces intense local vasoconstriction, slows systemic absorption of lidocaine, and thus reduces peak serum lidocaine concentrations, which reduces the risk of systemic lidocaine toxicity. The removal of a significant volume of tumescent subcutaneous fat by liposuction removes a significant portion of the tumescent lidocaine before it is absorbed into the systemic circulation. The threshold serum concentration for mild lidocaine toxicity (lightheadedness, paresthesias, tinnitus, blurred vision, nystagmus, ataxia, slurred speech, confusion) is 6 μg/mL.30–32
The principal aim of our research was to measure serum lidocaine concentrations as a function of milligram per kilo- gram dosage of tumescent lidocaine. Our main hypothesis was that dosages of tumescent lidocaine that are considerably larger than 7 mg/kg are a non significant risk of harm to patients.
The research had 4 specific aims. The first specific aim was to measure sequential serum lidocaine concentrations over 24 hours for each subject after subcutaneous infiltration of TLA on 3 separate occasions where the initial infiltrations were followed by no liposuction and the last infiltration was followed by liposuction. It has been suggested that IV lidocaine may have beneficial perioperative effects.33–37 We hypothesized that tumescent infiltration without liposuction produces a serum lidocaine concentration-time profile resembling a constant IV infusion lasting 12 to 16 hours or more. Furthermore, we hypothesized that liposuction removes significant amounts of lidocaine before it can be systemically absorbed. If the later hypothesis is true, then lidocaine data derived from liposuction patients cannot be used to establish the maximum recommended milligram per kilogram dosage of tumescent lidocaine for surgical procedures that do not involve liposuction.
The second aim was to record heart rate associated with doses of tumescent epinephrine and document adverse signs or symptoms associated with serum lidocaine concentrations. We hypothesized that tachycardia is uncommon and that adverse events associated with the large dosages of tumescent lidocaine and epinephrine are uncommon.
The third aim was to analyze the association between the milligram per kilogram dosage of tumescent lidocaine and subsequent peak serum lidocaine concentrations (Cmax) both without and with liposuction. We hypothesized that there is a linear relationship between the milligram per kilo- gram dosage of tumescent lidocaine and Cmax. Such a linear relationship would allow one to estimate Cmax as a function of milligram per kilogram dosage of tumescent lidocaine.
The fourth aim was to use tolerance interval analysis to calculate the proportion of individuals who, when given a specified milligram per kilogram dosage of tumescent lidocaine, will have a Cmax exceeding 6 μg/mL. We hypothesized that there are dosages larger than 7 mg/kg that are associated with a risk of mild lidocaine toxicity (Cmax ≥ 6 μg/mL) of <1/1000 and therefore are a non significant risk of harm to patients.
METHODS
This research was supported by the authors and registered at clinicaltrials.gov: NCT00977028. Before every procedure, subjects signed written informed consent approved by an IRB.
Inclusion criteria were ASA physical status I or II, no use of drugs that inhibit platelet function or inhibit the hepatic microsomal enzymes cytochrome P450 (CYP1A2 or CYP3A4) responsible for lidocaine metabolism, no clinical evidence of infection, and a negative urine pregnancy test. Prospective subjects had to first request liposuction before being informed of the opportunity to participate in this research. Participants were offered liposuction at no charge. Individual subjects served as their own controls. Large volume (≥500 mL) tumescent infiltration was accomplished using a peristaltic tumescent infiltration pump (HKSurgical. com, San Clemente, CA). Subcutaneous tumescent infiltra- tion was initiated by briefly using a spinal needle (20 gauge × 8.5 cm) to infiltrate a relatively small volume of tumescent lidocaine solution sufficient to allow subsequent painless insertion of blunt-tipped (16 gauge × 15 cm) multiorifice tumescent infiltration cannulas.
The anatomic area targeted for infiltration was constant for each subject. These areas, which varied among subjects, included abdomen, outer thigh, hips, back, inner thighs and knees, and female breasts. To minimize the chronotropic effects of epinephrine, most patients received oral clonidine 0.1 mg before tumescent infiltration. Clonidine (0.1 mg) and/or lorazepam (1 mg) by mouth 15 minutes before infiltration counteracted the tachycardia associated with epinephrine and provided mild anxiolysis and sedation. No narcotic analgesia or parenteral sedation was used. Prophylactic atropine, 0.3 mg IV or IM was administered to subjects with a history of syncope or near-syncope.
Each tumescent lidocaine infiltration procedure was followed by sequential serum lidocaine samples and clinical status evaluations at times T = 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, and 24 hours beginning immediately upon completion of infiltration.
For the 24 hours after infiltration, whenever serum samples were obtained, the awake patients were evaluated for any unpleasant subjective symptoms or signs of mild toxicity, including: lightheadedness, perioral numbness or nausea, tinnitus, blurred vision, nystagmus, ataxia, slurred speech, or confusion.
Patient monitoring during the first 12 to 14 hours included continuous cardiac rhythm, heart rate, pulse oximetry, and automatic arterial blood pressure. Heart rates, before, during, and after tumescent infiltration and immediately after liposuction were compared.
Serum samples were obtained from a peripheral vein using an indwelling 20-gauge IV catheter by a 2-syringe sampling technique. The first syringe contained 2 mL saline to flush the IV catheter and then remove and discard 2 mL of blood. Next, 10 mL blood was collected in a second syringe for assay of lidocaine by high-performance liquid chromatography by NMS Labs, Willow Grove, PA.38 The catheter was then flushed with 1 mL heparin 10 USP units per milliliter.
For each subject, the initial infiltration procedures were done without subsequent liposuction, and the final tumescent infiltration was followed by liposuction after allowing at least 1 hour of detumescence for gradual dispersion of subcutaneous tumescent fluid. Tumescent infiltration pro- cedures were separated by at least 1 week. The liposuction aspirate was collected in clear plastic volumetric canisters. After allowing at least 1 hour for gravitational separation of the lipid and aqueous aspirate, the resulting supernatant fat, infranatant blood-tinged tumescent anesthetic solution, and the total aspirate volume were recorded.
Serum lidocaine concentrations as a function of time, Cmax, the time when Cmax occurred (Tmax), and Cmax as a function of milligram per kilogram dosage of lidocaine were determined. Area under the curve (AUC∞) of serum lidocaine concentration-time profile was calculated by the trapezoid method. AUC∞, Cmax, and Tmax without and with liposuction were compared by the paired t test.
In some individual subjects, the lidocaine concentration (mg/L) in the TLA solutions and the lidocaine dosage (mg/kg) varied between procedures to achieve a targeted milligram per kilogram dosage of lidocaine and to have suf- ficient volume of TLA solution to accomplish liposuction of the area.
The choices of the milligram per kilogram dosages used in the present research were motivated by clinical experience with tumescent liposuction totally by local anesthesia. Worldwide experience with tumescent liposuction has shown that 45 mg/kg with liposuction is quite safe. Without liposuction, the range of safe dosages is not known.
The Cmax following 35 mg/kg without liposuction in the first 2 subjects was well below the toxic threshold of 6 μg/mL. These results provided pharmacokinetic assurance that 45 mg/kg without liposuction would not represent a significant risk of harm to the subjects.
To achieve an adequate range of input data for linear regression analysis, some of the subjects who received 45 mg/kg without liposuction also received 22.5 mg/kg (half of 45 mg/kg) in the second study without liposuction.
Statistical Analysis
We analyzed the data. The effect of liposuction on the systemic bioavailability of subcutaneous tumescent lidocaine was assessed by pairwise comparison of AUC∞s (paired t test) among subjects whose individual dosages of tumescent lidocaine were the same without and with liposuction. To assure statistical independence of these observations when comparing AUC∞ without and with liposuction, if a subject had 2 tumescent infiltration procedures without liposuction, then only 1 AUC∞ measurement was used in the paired t test. When these 2 lidocaine doses without liposuction were not equal, then we chose the dose that was the same as the dose with liposuction. If a subject’s 2 tumescent lidocaine doses without liposuction both equaled the dosage with liposuction, then we chose the smaller AUC∞ without liposuction. Because liposuction removes lidocaine before it can be absorbed systemically, the AUC∞ without liposuction is likely to be larger than the AUC∞ with liposuction. The choice of the smaller AUC∞ without liposuction was conservative, in the sense that it reduced the likelihood that the paired t test comparing AUC∞ without and with liposuction would incorrectly detect a significant difference between a subject’s AUC∞s (type I error).
For linear regression analysis of Cmax as a function of milligram per kilogram lidocaine dosage, only 1 of the 2 dosages without liposuction was used to assure statistical independence of observations. When the milligram per kilogram doses of lidocaine without liposuction were not equal, then the smallest of the 2 doses of lidocaine was used in the linear regression analysis to maximize the range of milligram per kilogram doses. When the milligram per kilogram doses of lidocaine without liposuction were equal, then the largest of the 2 Cmax values was chosen. The choice of the larger Cmax is conservative, in the sense that it increased the estimated probability that any given milligram per kilogram dose would produce a Cmax ≥6 μg/mL.
We used tolerance interval analysis to estimate the probability that a future milligram per kilogram dosage of tumescent lidocaine given to an individual would result in a Cmax ≥6 μg/mL.39–41 Tolerance intervals were calculated at a 99% level of confidence.
Supplemental Digital Content 1 (http://links.lww.com/ AA/B335) contains safety tips, and information regarding clinical lidocaine toxicity, case reports of tumescent lido- caine toxicity, tumescent lidocaine pharmacokinetics, for- mulation of TLA solution, tumescent infiltration techniques, detumescence, technique for calculating AUC∞, tolerance intervals, and R-Code to compute tolerance intervals.
Supplemental Digital Content 2 (http://links.lww.com/ AA/B336) is a video of the technique for painless subcutaneous infiltration of large volumes of tumescent lidocaine.
RESULTS
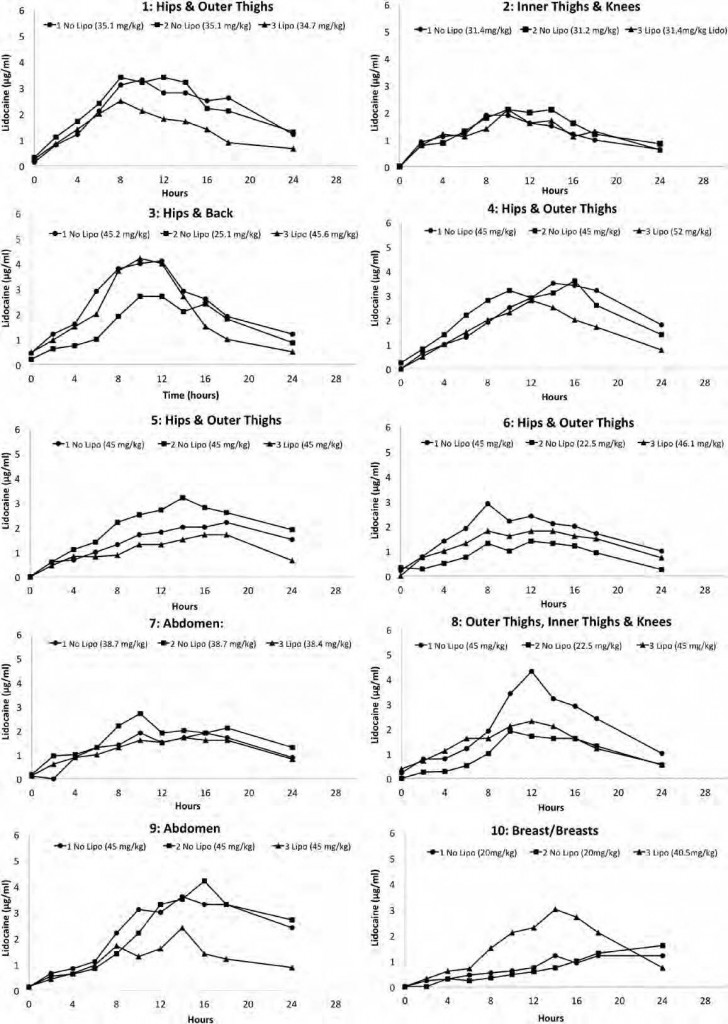
There were 41 TLA infiltration procedures. With 1 exception, all subjects had at least 2 tumescent infiltration procedures without subsequent liposuction and then 1 infiltration followed by liposuction. A single subject participated in only 1 TLA infiltration procedure without liposuction. All but 1 subject received the same milligram per kilogram dose of lidocaine at least once without liposuction and once with liposuction. The lidocaine concentration-time profile for each of the 14 subjects is shown in Figure 1. No subject experienced a peak serum lidocaine concentration larger than 4.4 μg/mL. Tables 1 and 2 present lidocaine dosage data, without and with liposuction, respectively.
Without liposuction, the range of lidocaine content in bags of tumescent solution was 700 to 1000 mg/bag. With liposuction, the range of lidocaine content was 770 to 1000 mg/bag. The ranges of milligram per kilogram dosages of lidocaine were 19.2 to 45.0 mg/kg without liposuction and 19.4 to 52 mg/kg with liposuction. Ten subjects received 45 mg/kg without liposuction and at least 45 mg/kg with liposuction. The total milligram dose of tumescent lidocaine ranged from 1800mg to 3600mg. During this research, the volume of infiltrated TLA solution ranged from 2 to 4 L. Subjects received no IV fluids, no systemic sedatives, and no narcotic analgesics.
Among those who received 45 mg/kg tumescent lido- caine for liposuction, the mean total volume of aspirate was 2416 mL (range, 1525–3300 mL), mean volume of super- natant fat was 1863 mL (range, 1250–2900 mL), and mean volume infranatant blood-tinged anesthetic solution was 553 mL (range, 130–1100 mL).
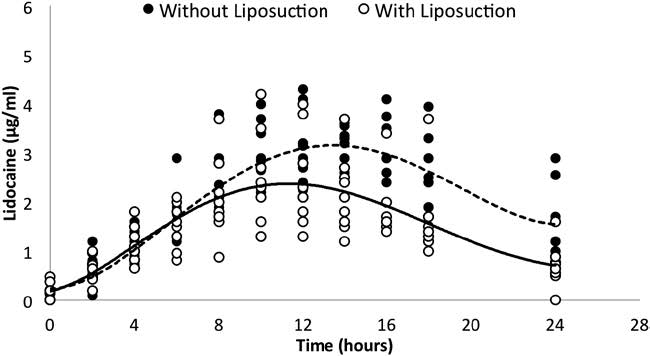
At equal milligram per kilogram dosages of tumescent lidocaine without and with liposuction, the mean AUC∞ for serum lidocaine concentration-time profile without lipo- suction (56.2 μg·h/mL) was significantly higher than that with liposuction (40.7 μg·h/mL; P = 0.001). As presented in Figure 2, liposuction removed approximately 28% of the lidocaine before it could be absorbed into the systemic circulation. At equal milligram per kilogram dosages of tumescent lidocaine, the mean Cmax without liposuction 2.9 μg/mL (range, 1.2–4.4) was significantly higher than the mean Cmax with liposuction 2.38 μg/mL (range, 0.97–3.8) by the paired t test (P = 0.001). The mean Tmax without liposuction was 13.1 hours (range, 8–24), which was not significantly different from the mean Tmax with liposuction 12.5 hours (range, 8–18; P = 0.19).
Without liposuction, the dose of epinephrine ranged from 1.2 to 4.3 mg and the mean difference in heart rate before and after infiltration was −3.4 (range, −24 to +17). With liposuction, the dose of epinephrine ranged from 1.6 to 4.3 mg, and the difference in heart rate before infiltration and after liposuction was not significant (P = 0.13; mean = +5; range, −12 to +33).
One subject who was relatively thin, with body mass index of 20, received 45 mg/kg without liposuction, which produced a Cmax of 4.3 μg/mL and experienced transient nausea approximately 12 hours after infiltration. There were no other lidocaine-associated adverse events.
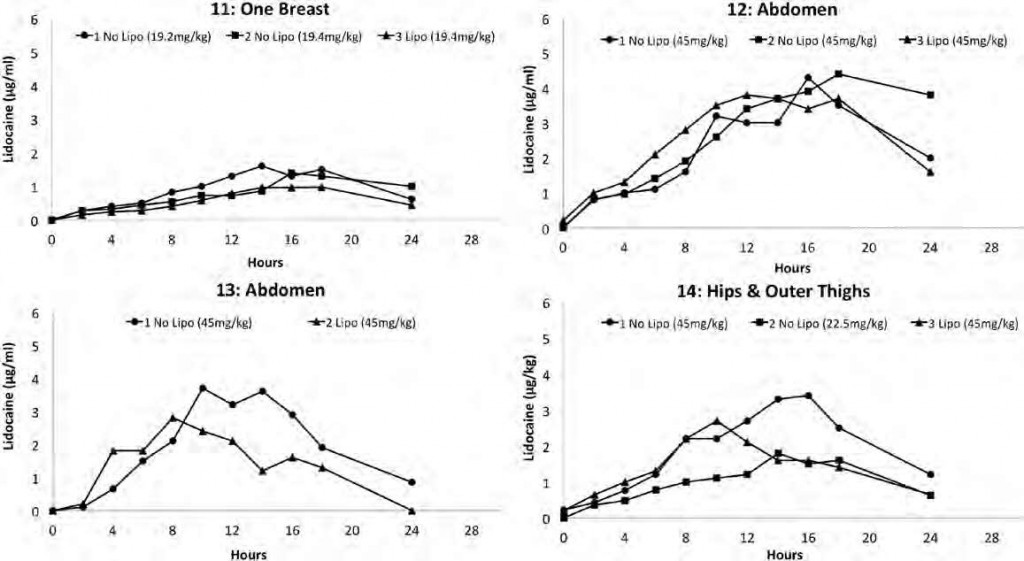
Figure 1. Serum lidocaine concentrations over time for each of the 14 subjects after subcutaneous infiltration of tumescent lidocaine anes- thesia. Subject number and anatomic area of infiltration are presented on the top of each plot. The figure legend presents whether or not liposuction was done after tumescent infiltration indicated by “No Lipo” and “Lipo,” respectively, and the tumescent lidocaine dosage (mg/kg).
There was no clinical evidence of epinephrine toxicity, such as chest pain or discomfort, dyspnea, dizziness, headache, anxiety, nervousness, restlessness, tremors, diaphoresis, pallor, rapid, irregular or pounding heart rate, or pounding in the ears.
There were no observed signs or symptoms of neurotoxicity, syncope, and near-syncope. There was no evidence of cardiac toxicity, such as arrhythmia, tachycardia, bradycardia, hypertension, hypotension, volume overload heart failure, pulmonary edema, or hypoxia.
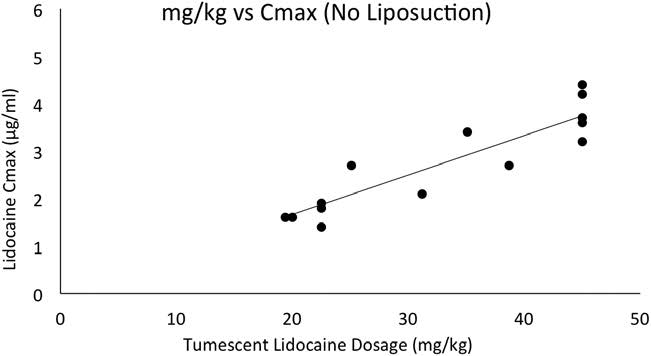
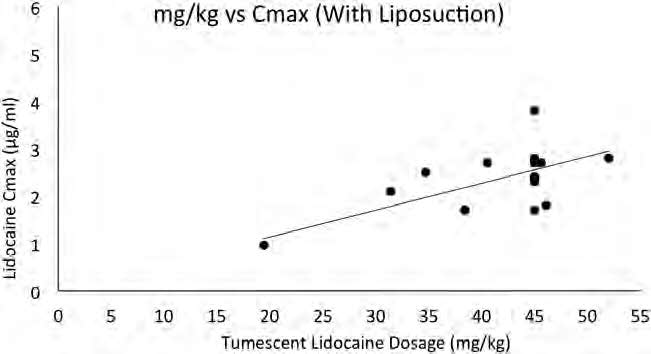
Without liposuction, there was a strong linear relationship between milligram per kilogram dosage of tumescent lidocaine and Cmax (R2 = 0.85; Fig. 3). With liposuction, there was a weaker linear relationship between milligram per kilogram dosage of tumescent lidocaine and Cmax (R2 = 0.36; see Fig. 4).
Based on the tolerance interval analysis, the estimated probability that a future milligram per kilogram dose of tumescent lidocaine given to an individual would result in a Cmax ≥6 μg/mL is shown in Table 3.
Supplemental Digital Content 3 (http://links.lww.com/ AA/B337) presents patient-level raw data and additional analysis including tables in comma-separated values (cvs) format.
DISCUSSION
Our findings confirmed our main hypothesis that doses of TLAthat are far larger than the current FDAlimit of 7 mg/kg are a nonsignificant risk of harm to patients.
After the subcutaneous infiltration of tumescent lidocaine, we observed the serum concentration-time profiles without and with liposuction and found that tumescent lidocaine absorption continues beyond 24 hours. For a given dosage of a drug, prolonging its systemic absorption reduces its Cmax. This explains the remarkable safety of large dosages of tumescent lidocaine and epinephrine.
At equal doses of tumescent lidocaine, the average AUC∞ of the concentration-time profiles is 28% smaller with liposuction than it is without liposuction. This supports our hypothesis that liposuction removes a significant amount of subcutaneous tumescent lidocaine before it can be absorbed into the circulation. Thus, data derived from liposuction patients cannot be used to estimate the maximal safe milligram per kilogram dosage of tumescent lidocaine without liposuction.
Furthermore, these concentration-time profiles resemble the profile of a constant IV lidocaine infusion that is discontinued at Tmax. There is a growing literature indicating that systemic IV lidocaine may have beneficial perioperative effects, including preemptive analgesia, reduced postoperative narcotic requirements, and reduced systemic inflammatory response to surgical trauma.33,34,42–47 The observation that tumescent infiltration produces a concentration-time profile similar to a constant IV infusion of lidocaine suggests a new hypothesis, to be tested in the future, that local TLA may have desirable systemic effects.
During each of the 41 studies, we observed heart rate, arterial blood pressure, pulse oximetry, and cardiac rhythm and inquired about any subjective symptoms suggestive of lidocaine toxicity. There were no episodes of tachycardia although most patients did receive oral clonidine (0.1 mg) for its anxiolytic effect and to counteract the positive chronotropic effects of epinephrine. One patient encountered a brief episode of nausea at 45mg/kg without liposuction. The data indicates that without liposuction 45mg/kg is risky, while 28mg/kg is a more reasonable maximal safe dosage. Otherwise, careful observation of patients over the course of 41 pharmacokinetic studies revealed no adverse events associated with the systemic effects of lidocaine and epinephrine. This finding confirmed our hypothesis that adverse events associated with the large dosages of tumescent lidocaine with epinephrine are infrequent.
| Table 1.Demographic Data Without Liposuction | ||||||||||||||
| Patient no.- | Body | Lido | Clonidine | Atropine | Lido | BMI | ||||||||
| study no. | area | (mg/bag) | Epi (mg/L) | (mg) | (mg) | Wt (kg) | Ht (m) | m2 | Lido (mg) | (mg/kg) | (kg/m2) | Cmax | Tmax | AUC∞ |
| 01-1 | H-OT | 700 | 1 | 0 | 0.3 | 59.7 | 1.59 | 2.53 | 2100 | 35.1 | 23.6 | 3.3 | 10 | 54.5 |
| 01-2 | H-OT | 700 | 0.5 | 0 | 0.3 | 59.7 | 1.59 | 2.53 | 2100 | 35.1 | 23.6 | 3.4 | 8 | 57.7 |
| 02-1 | I(T/K) | 700 | 1 | 0 | 0 | 62.1 | 1.68 | 2.81 | 1956 | 31.4 | 22.1 | 1.9 | 8 | 29.4 |
| 02-2 | I(T/K) | 700 | 0.5 | 0 | 0 | 62.6 | 1.68 | 2.81 | 1956 | 31.2 | 22.3 | 2.1 | 10 | 35 |
| 03-1 | H-F | 1000 | 1 | 0 | 0 | 83.6 | 1.78 | 3.17 | 3782 | 45.2 | 26.4 | 4.1 | 12 | 61.5 |
| 03-2 | Rt:H-F | 1000 | 1 | 0 | 0 | 82.7 | 1.78 | 3.17 | 2073 | 25.1 | 26.1 | 2.7 | 11 | 42 |
| 04-1 | H-OT | 1000 | 1 | 0.1 | 0 | 70.2 | 1.625 | 2.64 | 3159 | 45 | 26.6 | 3.5 | 14 | 57.9 |
| 04-2 | H-OT | 1000 | 0.5 | 0.1 | 0 | 70.2 | 1.625 | 2.64 | 3171 | 44.9 | 26.6 | 3.6 | 16 | 59 |
| 05-1 | H-OT | 800 | 1 | 0.1 | 0 | 74.8 | 1.75 | 3.06 | 3375 | 45 | 24.5 | 2.2 | 18 | 44.5 |
| 05-2 | H-OT | 800 | 1 | 0.1 | 0 | 75.5 | 1.75 | 3.06 | 3406 | 45 | 24.7 | 3.2 | 14 | 60.5 |
| 06-1 | H-OT | 1000 | 1 | 0.1 | 0.3 | 68.5 | 1.69 | 2.85 | 3090 | 45 | 24 | 2.9 | 8 | 44.4 |
| 06-2 | H-OT | 1000 | 1 | 0.1 | 0.3 | 68.6 | 1.69 | 2.85 | 1539 | 22.5 | 24.1 | 1.4 | 12 | 21 |
| 07-1 | Abd | 1000 | 1 | 0.1 | 0 | 64.9 | 1.72 | 2.96 | 2514 | 38.7 | 21.9 | 1.9 | 10 | 33.4 |
| 07-2 | Abd | 1000 | 0.5 | 0.1 | 0 | 65.3 | 1.72 | 2.96 | 2531 | 38.7 | 22.1 | 2.7 | 10 | 44.3 |
| 08-1 | 2 OT, I(T/K) | 1000 | 1 | 0 | 0 | 55.9 | 1.67 | 2.79 | 2516 | 45 | 20 | 4.3 | 12 | 52.7 |
| 08-2 | 1 OT, I(T/K) | 1000 | 1 | 0 | 0 | 54.1 | 1.67 | 2.79 | 1217 | 22.5 | 19.4 | 1.9 | 10 | 26 |
| 09-1 | Abd | 1000 | 1 | 0.1 | 0 | 70.76 | 1.6 | 2.56 | 3189 | 45 | 27.6 | 3.6 | 14 | 70.3 |
| 09-2 | Abd | 1000 | 1 | 0.1 | 0 | 70.76 | 1.6 | 2.56 | 3189 | 45 | 27.6 | 4.2 | 16 | 70.8 |
| 10-1 | L Brst | 1000 | 1 | 0.1 | 0 | 100 | 1.73 | 2.99 | 2018 | 20 | 33.4 | 1.2 | 14 | 25.6 |
| 10-2 | L Brst | 1000 | 1 | 0.1 | 0 | 100 | 1.73 | 2.99 | 2028 | 20 | 33.4 | 1.6 | 24 | 26.9 |
| 11-1 | L Brst | 1000 | 1 | 0.1 | 0 | 79.1 | 1.65 | 2.72 | 1522 | 19.2 | 29.1 | 1.6 | 14 | 24.2 |
| 11-2 | L Brst | 1000 | 1 | 0.1 | 0 | 80 | 1.65 | 2.72 | 1549 | 19.4 | 29.4 | 1.4 | 16 | 21.8 |
| 12-1 | Abd | 1000 | 1 | 0 | 0 | 80.7 | 1.575 | 2.48 | 3640 | 45 | 32.5 | 4.3 | 16 | 62 |
| 12-2 | Abd | 1000 | 1 | 0 | 0 | 81 | 1.575 | 2.48 | 3651 | 45 | 32.7 | 4.4 | 18 | 77.4 |
| 13-2 | H-OT | 1000 | 1 | 0 | 0 | 66.4 | 1.63 | 2.66 | 2957 | 44.5 | 25 | 3.7 | 10 | 48.3 |
| 14-1 | H-OT | 1000 | 1 | 0 | 0.3 | 76.4 | 1.75 | 3.06 | 3436 | 45 | 25 | 3.4 | 16 | 49.6 |
| 14-2 | H-OT | 1000 | 1 | 0 | 0.3 | 76.4 | 1.75 | 3.06 | 1718 | 22.5 | 25 | 1.8 | 14 | 26.5 |
The weight and height for each of the 14 subjects and the drug and dosage data for each of the 27 research studies including peak serum concentration (Cmax), time at Cmax (Tmax), and area under the curve of the serum lidocaine concentration-time profile (AUC∞) are given.
Abd = abdomen; BMI = body mass index; Epi = epinephrine; H-F = hips and flanks/back; Ht = height; H-OT = hips and outer thighs; I(T/K) = inner thighs and knees; L Brst = left breast; Lido = lidocaine; Wt = weight.
| Table 2. Demographic Data with Liposuction | |||||||||||||||||
| Patient
no.-study no. |
Area |
Lido (mg/bag) | Epi (mg/bag) | Clonidine (mg) | Atropine (mg) |
Wt (kg) |
Ht (m) |
m2 |
Lido (mg) | Lido (mg/kg) |
BMI |
Cmax |
Tmax |
AUC∞ |
Aspirate (mL) | Supranat (mL) | Infranat (mL) |
| 01-3 | H-OT | 700 | 1 | 0 | 0.3 | 59.7 | 1.59 | 2.53 | 2074 | 34.7 | 23.6 | 2.5 | 8 | 35.3 | 1950 | 1750 | 200 |
| 02-3 | I(T/K) | 700 | 1 | 0 | 0 | 63 | 167.7 | 2.81 | 1984 | 31.4 | 22.4 | 2.1 | 10 | 31 | 1100 | 750 | 350 |
| 03-3 | H-F | 1000 | 1 | 0 | 0 | 83.6 | 1.78 | 3.17 | 3900 | 46.7 | 26.3 | 4.2 | 10 | 48.6 | 1900 | 1250 | 650 |
| 04-3 | H-OT | 1000 | 1 | 0.1 | 0 | 70.2 | 1.625 2.64 | 3159 | 52 | 26.6 | 2.8 | 12 | 40.6 | 2425 | 2000 | 425 | |
| 05-3 | H-OT | 800 | 1 | 0.1 | 0 | 75.5 | 1.75 | 3.06 | 3405 | 45 | 24.7 | 1.7 | 16 | 27.6 | 2220 | 1845 | 525 |
| 06-3 | H-OT | 1000 | 1 | 0.1 | 0.3 | 69.1 | 1.69 | 2.85 | 3190 | 46.1 | 24.2 | 1.8 | 12 | 33.7 | 2080 | 1840 | 240 |
| 07-3 | Abd | 1000 | 1 | 0.1 | 0 | 66.2 | 1.72 | 2.96 | 2550 | 38.4 | 22.4 | 1.7 | 14 | 31.9 | 1300 | 950 | 350 |
| 08-3 | OT, I(T/K) | 1000 | 1 | 0 | 0.3 | 55.2 | 1.67 | 2.79 | 2516 | 45.6 | 19.8 | 2.3 | 12 | 34.8 | 1525 | 1395 | 130 |
| 09-3 | Abd | 1000 | 1 | 0.1 | 0 | 71.2 | 1.6 | 2.56 | 3318.6 | 46.6 | 27.8 | 2.4 | 14 | 33.8 | 2700 | 1875 | 825 |
| 10-3 | 2Brst | 1000 | 1 | 0.1 | 0 | 101 | 1.73 | 2.99 | 4122 | 40.5 | 33.8 | 2.7 | 16 | 37.7 | 2500 | 1450 | 1050 |
| 11-3 | L Brst | 1000 | 1 | 0.1 | 0 | 81.1 | 1.65 | 2.72 | 1572 | 19.4 | 29.8 | 0.97 | 18 | 15.2 | 700 | 450 | 250 |
| 12-3 | Abd | 1000 | 1 | 0.1 | 0 | 81.7 | 1.575 2.48 | 3674 | 45 | 32.9 | 3.8 | 12 | 67.8 | 2800 | 2260 | 540 | |
| 13-2 | H-OT | 1000 | 1 | 0 | 0 | 66.4 | 1.63 | 2.66 | 2993 | 45.7 | 25 | 2.8 | 8 | 33 | 2550 | 2200 | 350 |
| 14-3 | H-OT | 1000 | 1 | 0.1 | 0.3 | 76.4 | 1.75 | 3.06 | 3436 | 45 | 25 | 2.7 | 10 | 35.7 | 3300 | 2900 | 400 |
The weight and height for each of the 14 subjects and the drug and dosage data for each of the 14 research studies including peak serum concentration (Cmax), time at Cmax (Tmax), area under the curve of the serum lidocaine concentration-time profile (AUC∞) are given.
Abd = abdomen; BMI = body mass index; Epi = epinephrine; H-F = hips and flanks/back; H-OT = hips & and outer thighs; Ht = height; Infranat = infranatant; I(T/K) = inner thighs and knees; L Brst = left breast; Lido = lidocaine; Supranat = supernatant; Wt = weight.
Figure 2. Comparison of serum lidocaine concentrations at sequential times over 24 h following 45 mg/kg tumescent lidocaine, without liposuction (closed circles) and with liposuction (open circles). The AUC∞ of the mean concentrations (solid line) at each time point without liposuction (56.2 μg·h/mL) is 28% greater than the AUC∞ of the mean concentrations (dashed line) with liposuction (40.7 μg·h/mL).
Figure 3. Scatter plot of tumescent lidocaine dosage versus peak serum lidocaine concentrations (Cmax) without liposuction. The
solid line represents the line of regression with a coefficient of determination (R2) of 0.85.
Figure 4. Scatter plot of tumescent lidocaine dosage versus peak serum lidocaine concentrations (Cmax) with liposuction. The solid
line represents the line of regression with a coefficient of determination (R2) of 0.35.
The association between the milligram per kilogram dosage of tumescent lidocaine and the subsequent peak serum lidocaine concentrations (Cmax) was analyzed both without and with liposuction. The data confirmed our hypothesis that there is a close linear relationship between the milligram per kilogram dosage of tumescent lidocaine without liposuction and Cmax. Thus, an increased milligram per kilogram dosage of tumescent lidocaine is associated with an increased risk of toxicity.
| Table 3. Risk of Lidocaine Serum Concentration >6 μg/mL (99% Confidence) | |||||||
|
No liposuction |
Dosage of tumescent lidocaine (mg/kg) | ||||||
| 21 | 28 | 35 | 40 | 45 | 50 | 55 | |
| < 1/1016 | 1/5 × 106 | 1/10,000 | 1/750 | 1/80 | 1/15 | 1/4 | |
| 1/5 × 1010 | 1/2 × 107 | 1/2 × 105 | 1/15,000 | 1/2000 | 1/500 | 1/100 | |
Liposuction removes lidocaine before it can be absorbed and thus reduces the correlation between the milligram per kilogram dosage of tumescent lidocaine liposuction and Cmax. With liposuction, an estimate of the maximum safe dosage of tumescent lidocaine is less reliable than without liposuction. Years of worldwide experience have shown that 55 mg/kg tumescent lidocaine for liposuction is remarkably safe.48,49 This dosage is safe most of the time. Multiple large surveys involving thousands of procedures have found no evidence of tumescent lidocaine toxicity at recommended dosages.50–52 However, 55 mg/kg may be too risky if lidocaine absorption is too rapid (failure to add epinephrine to the solution of tumescent lidocaine) or if lidocaine metabolism is too slow (diabetes,53 adverse interactions with drugs that inhibit the hepatic microsomal isoenzymes cytochrome P450 3A4 and 1A2 such as erythromycin,54 sertraline, fluconazole or ciprofloxacin, propofol,55 or general anesthesia56) or if patients have very low serum protein concentrations or if surgery is cancelled before liposuction can be completed. Based on the present data and considerable worldwide experience, we believe that 45 mg/kg is a safe and prudent maximum dosage of tumescent lidocaine for liposuction. Furthermore, 45 mg/kg is less likely than 55 mg/kg to permit excessive amounts of liposuction.
Tolerance interval analysis was used to calculate the proportion of individuals who, when given a specified milligram per kilogram dosage of tumescent lidocaine, will have a Cmax exceeding 6 μg/mL. The results confirmed our hypothesis that dosages larger than 7 mg/kg are associated with a risk of <1/1000 for mild lidocaine toxicity. In particular, without liposuction, a dosage of 45 mg/kg has an estimated risk of mild toxicity of 1/80 and at 28 mg/kg the estimated risk of mild toxicity was several orders of magnitude <1/2000. With liposuction, a dosage of 45 mg/kg has an estimated risk of mild toxic- ity of 1/2000. Thus, the risk of mild toxicity at 28 mg/kg without liposuction and 45 mg/kg with liposuction is each <1/1000 and can be said to represent a non significant risk of harm to patients. For nonliposuction surgeries, 28 mg/kg tumescent lidocaine is a prudent choice while allowing at least 2 L tumescent solution in a 70-kg adult.
Adverse Events with Tumescent Anesthesia
All reported adverse events associated with TLA have been the result of clinician error, such as inadvertent IV delivery of tumescent solution,57 miscommunication leading to excessive lidocaine in the tumescent lidocaine solution, unawareness of drug interactions that reduce lidocaine metabolism by impairing cytochrome P450 1A2 and 3A4,58 and ad libitum formulations of tumescent solutions using bupivacaine, mepivacaine, or triamcinolone59 (Fig. 5).
In 1999, an influential report of 4 liposuction fatalities concluded that, “Tumescent liposuction can be fatal; perhaps in part because of lidocaine toxicity or lidocaine- related drug interactions.”60 All 4 patients received general anesthesia or heavy IV sedation. The tumescent lidocaine dosages were 10, 14.3, 31.4, and 40 mg/kg. Our data suggest that it was unlikely that tumescent lidocaine caused toxicity in these cases.
Study Limitations
Our estimates are preliminary and based on a sample of only 14 subjects. A larger sample size would provide both more evidence of the validity of our normality assumptions and more reliable tolerance interval estimates. Further proof of safety requires a randomized clinical trial involving patients with a wide range of clinical problems. Therapeutic surgeries without liposuction are likely to involve patients who are less healthy and who take more medications than do healthy liposuction patients. Unrecognized comorbidities and unanticipated clinical situations may be encountered. A patient may be taking a drug that impairs lidocaine metabolism and increases the risk of lidocaine toxicity. Pediatric patients, geriatric patients with impaired cardiac function, and patients with low or very high body mass index might not have the same pharmacokinetic response to tumescent lidocaine as a healthy adult. Any clinical condition associated with slower lidocaine metabolism or faster lidocaine absorption requires a reduced lidocaine milligram per kilogram dosage.
The choice of a recommended maximum safe milligram per kilogram dosage should be tempered by the realization that all statistical estimates are affected by sampling error. Careful clinical judgment must influence the choice of maximum permissible dosage for an individual patient. Dilute tumescent lidocaine is safer than undiluted commercial (0.5%, 1%, and 2%) solutions. When using undiluted commercial lidocaine with epinephrine, the traditional 7 mg/kg dosage limit should be observed.
We performed subcutaneous infiltration of TLA using a specific technique and specially designed infiltration cannulas. Different techniques and different clinicians may have different results.
CONCLUSIONS
Within our sample of 14 subjects there was no evidence of lidocaine or epinephrine toxicity. Preliminary estimates for maximum safe dosages of tumescent lidocaine are 28 mg/kg without liposuction and 45mg/kg with liposuction. As a result of delayed systemic absorption, these dosages yield serum lidocaine concentrations below levels associated with mild toxicity and represent a non significant risk of harm to patients.
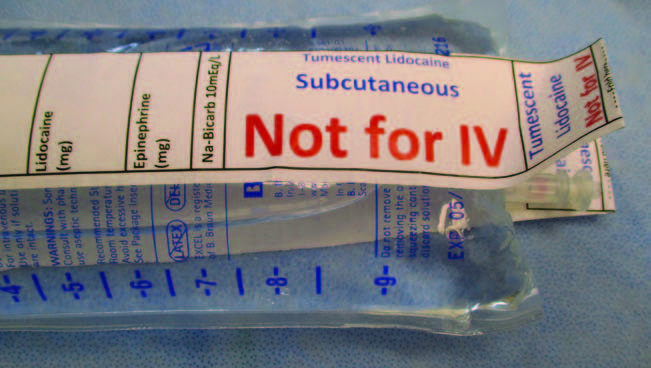
Figure 5. “Safety Label” applied to a bag tumescent lidocaine solution. The label overhangs the port for the IV tubing spike. A “Safety Label” is a visual reminder that the bag contains tumescent lidocaine for subcutaneous delivery and is not for IV delivery.
From the *Department of Dermatology, University of California, Irvine, Medical Sciences, Irvine, California; and †Department of Statistics, University of California, Riverside, Riverside, California.
Accepted for publication May 8, 2015.
Funding: This was an investigator-initiated research funded entirely by the authors.
Conflict of Interest: See Disclosures at the end of the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.anesthesia-analgesia.org).
Reprints will not be available from the authors.
Address correspondence to Jeffrey A. Klein, MD, MPH, 30280 Rancho Viejo Rd., San Juan Capistrano, CA 92675. Address e-mail to jeff@kleinmd.com.
Copyright © 2016 International Anesthesia Research Society. This is an open- access article distributed under the terms of the Creative Commons Attribu- tion-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND), where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially.
DOI: 10.1213/ANE.0000000000001119
DISCLOSURES
Name: Jeffrey A. Klein, MD, MPH.
Contribution: This author helped with the experimental design, conducted the clinical study, and prepared the manuscript.
Attestation: Jeffrey A. Klein approved the final manuscript. Dr. Klein attests to the integrity of the original data and analysis reported in the final manuscript. Dr. Klein is the archival author.
Conflicts of Interest: Jeffrey A. Klein owns patents on devices for tumescent infiltration. Dr. Klein’s wife owns HK Surgical, Inc., a company that sells devices for delivering tumescent anesthesia.
Name: Daniel R. Jeske, PhD.
Contribution: This author helped with the experimental design and the statistical analysis.
Attestation: Daniel R. Jeske approved the final manuscript. Dr. Jeske attests to the integrity of the original data and analysis reported in the final manuscript.
Conflicts of Interest: The author has no conflicts of interest to declare.
This manuscript was handled by: Ken B. Johnson, MD.
REFERENCES
- Klein JA. The tumescent technique for liposuction J Am Acad Cosmetic Surg 1987;4:263–7
- Klein JA. Tumescent technique for local anesthesia improves safety in large-volume liposuction. Plast Reconstr Surg 1993;92:1085–98
- Shimizu Y, Nagasao T, Taneda H, Sakamoto Y, Asou T, Imanishi N, Kishi K. Combined usage of intercostal nerve block and tumescent anaesthesia: an effective anaesthesia technique for breast augmentation. J Plast Surg Hand Surg 2014;48:51–5
- Sleth JC, Servais R, Saizy [Tumescent infiltrative anaesthesia for mastectomy: about six cases]. Ann Fr Anesth Reanim 2008;27:941–4
- Orgill Excision and skin grafting of thermal burns. N Engl J Med 2009;360:893–901
- Bussolin L, Busoni P, Giorgi L, Crescioli M, Messeri Tumescent local anesthesia for the surgical treatment of burns and postburn sequelae in pediatric patients. Anesthesiology 2003;99:1371–5
- Gümüş Tumescent infiltration of lidocaine and adrenaline for burn surgery. Ann Burns Fire Disasters 2011;24:144–8
- Blome-Eberwein S, Abboud M, Lozano DD, Sharma R, Eid S, GogalEffect of subcutaneous epinephrine/saline/local anesthetic versus saline-only injection on split-thickness skin graft donor site perfusion, healing, and pain. J Burn Care Res 2013;34:e80–6
- Cohn MS, Seiger E, Goldman S. Ambulatory phlebectomy using the tumescent technique for local anesthesia. Dermatol Surg 1995;21:315–8
- Vuylsteke ME, Mordon Endovenous laser ablation: a review of mechanisms of action. Ann Vasc Surg 2012;26:424–33
- Barkmeier LD, Hood DB, Sumner DS, Mansour MA, Hodgson KJ, Mattos MA, Ramsey D. Local anesthesia for infrainguinal arterial reconstruction. Am J Surg 1997;174:202–4
- Bush RG, Hammond KA. Tumescent anesthetic technique for long saphenous stripping. J Am Coll Surg 1999;189:626–8
- Haines WY, Deets R, Lu N, Matsuura Tumescent anesthesia reduces pain associated with balloon angioplasty of hemodi- alysis fistulas. J Vasc Surg 2012;56:1453–6
- Behroozan DS, Goldberg Dermal tumescent local anesthesia in cutaneous surgery. J Am Acad Dermatol 2005;53:828–30
- Girard C, Debu A, Bessis D, Blatière V, Dereure O, Guillot Treatment of Gorlin syndrome (nevoid basal cell carci- noma syndrome) with methylaminolevulinate photodynamic therapy in seven patients, including two children: interest of tumescent anesthesia for pain control in children. J Eur Acad Dermatol Venereol 2013;27:e171–5
- Kendler M, Micheluzzi M, Wetzig T, Simon Electrochemotherapy under tumescent local anesthesia for treat- ment of cutaneous metastases. Dermatolog Surg. 2013;39:1023–32
- Stoffels I, Dissemond J, Schulz A, Hillen U, Schadendorf D, Klode J. Reliability and cost-effectiveness of complete lymph node dissection under tumescent local anaesthesia vs. general anaesthesia: a retrospective analysis in patients with malig- nant melanoma AJCC stage III. J Eur Acad Dermatol Venereol 2012;26:200–6
- Ramon Y, Barak Y, Ullmann Y, Hoffer E, Yarhi D, Bentur Pharmacokinetics of high-dose diluted lidocaine in local anesthesia for facelift procedures. Ther Drug Monit 2007;29:644–7
- Abramson DL. Tumescent abdominoplasty: an ambulatory office procedure. Aesthetic Plast Surg 1998;22:404–7
- Narita M, Sakano S, Okamoto S, Uemoto S, Yamamoto M. Tumescent local anesthesia in inguinal herniorrhaphy with a PROLENE hernia system: original technique and results. Am J Surg 2009;198:e27–31
- Kayaalp C, Olmez A, Aydin C, Piskin Tumescent local anes- thesia for excision and flap procedures in treatment of pilonidal disease. Dis Colon Rectum 2009;52:1780–3
- Locke M, Windsor J, Dunbar Human adipose-derived stem cells: isolation, characterization and applications in surgery. ANZ J Surg 2009;79:235–44
- Prasetyono Tourniquet-Free Hand Surgery Using the One-per-Mil Tumescent Technique. Arch Plast Surg 2013;40:129–33
- Mizukami T, Hamamoto M. Tumescent local anesthesia for a revascularization of a coronary subclavian steal Ann Thorac Cardiovasc Surg 2007;13:352–4
- Carlson Total mastectomy under local anesthesia: the tumescent technique. Breast J 2005;11:100–2
- Rosenberg PH, Veering BT, Urmey Maximum recom- mended doses of local anesthetics: a multifactorial concept. Reg Anesth Pain Med 2004;29:564–75
- Scott “Maximum recommended doses” of local anaesthetic drugs. Br J Anaesth 1989;63:373–4
- Coldiron B, Coleman III WP, Cox SE, Jacob C, Lawrence N, Kaminer M, Narins RS. ASDS guidelines of care for tumescent liposuction. Dermatol Surg 206;32:709–16
- McKay W, Morris R, Mushlin Sodium bicarbonate attenuates pain on skin infiltration with lidocaine, with or without epi- nephrine. Anesth Analg 1987;66:572–4
- Gianelly R, von der Groeben JO, Spivack AP, Harrison DC. Effect of lidocaine on ventricular arrhythmias in patients with coronary heart disease. N Engl J Med 1967;277:1215–9
- Scott DB. Evaluation of the toxicity of local anaesthetic agents in man. Br J Anaesth 1975;47:56–61
- Rosaeg OP, Bell M, Cicutti NJ, Dennehy KC, Lui AC, Krepski Pre-incision infiltration with lidocaine reduces pain and opioid consumption after reduction mammoplasty. Reg Anesth Pain Med 1998;23:575–9
- Garutti I, Rancan L, Simón C, Cusati G, Sanchez-Pedrosa G, Moraga F, Olmedilla L, Lopez-Gil MT, Vara Intravenous lidocaine decreases tumor necrosis factor alpha expression both locally and systemically in pigs undergoing lung resection sur- gery. Anesth Analg 2014;119:815–28
- De Oliveira GS Jr, Fitzgerald P, Streicher LF, Marcus RJ, McCarthy RJ. Systemic lidocaine to improve postoperative quality of recovery after ambulatory laparoscopic Anesth Analg 2012;115:262–7
- Yon JH, Choi GJ, Kang H, Park JM, Yang Intraoperative systemic lidocaine for pre-emptive analgesics in subtotal gas- trectomy: a prospective, randomized, double-blind, placebo- controlled study. Can J Surg 2014;57:175–82
- Kim KT, Cho DC, Sung JK, Kim YB, Kang H, Song KS, Choi Intraoperative systemic infusion of lidocaine reduces postoper- ative pain after lumbar surgery: a double-blinded, randomized, placebo-controlled clinical trial. Spine J 2014;14:1559–66
- Grigoras A, Lee P, Sattar F, Shorten G. Perioperative intrave- nous lidocaine decreases the incidence of persistent pain after breast Clin J Pain 2012;28:567–72
- Hill J, Roussin A, Lelorier J, Caille High-pressure liquid chro- matographic determination of lidocaine and its active deethyl- ated metabolites. J Pharm Sci 1980;69:1341–3
- Krishnamoorthy K, Mathew Statistical Tolerance Regions. Hoboken, NJ: John Wiley & Sons, Inc., 2009
- Hahn G, Meeker WQ. Statistical Intervals: A Guide for Practitioners. John Wiley & Sons, Inc., 1991
- Myhre J, Jeske DR, Rennie M, Bi Tolerance intervals in a heteroscedastic linear regression context with applications to Aerospace equipment surveillance. International J Quality Statistics Reliability 2009;2009:Article ID 126283, 8 pages
- Piegeler T, Votta-Velis EG, Liu G, Place AT, Schwartz DE, Beck-Schimmer B, Minshall RD, Borgeat A. Antimetastatic potential of amide-linked local anesthetics: inhibition of lung adenocarcinoma cell migration and inflammatory Src signal- ing independent of sodium channel Anesthesiology 2012;117:548–59
- McKay A, Gottschalk A, Ploppa A, Durieux ME, Groves DS. Systemic lidocaine decreased the perioperative opioid anal- gesic requirements but failed to reduce discharge time after ambulatory Anesth Analg 2009;109:1805–8
- de Klaver MJ, Buckingham MG, Rich Lidocaine attenu- ates cytokine-induced cell injury in endothelial and vascular smooth muscle cells. Anesth Analg 2003;97:465–70
- McCarthy GC, Megalla SA, Habib AS. Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery: a systematic review of randomized controlled trials. Drugs 2010;70:1149–63
- Wang HL, Zhang WH, Lei WF, Zhou CQ, Ye The inhibitory effect of lidocaine on the release of high mobility group box 1 in lipopolysaccharide-stimulated macrophages. Anesth Analg 2011;112:839–44
- Kaczmarek DJ, Herzog C, Larmann J, Gillmann HJ, Hildebrand R, Schmitz M, Westermann A, Harendza T, Werdehausen R, Osthaus AW, Echtermeyer F, Hahnenkamp K, Wollert KC, Theilmeier G. Lidocaine protects from myocardial damage due to ischemia and reperfusion in mice by its antiapoptotic Anesthesiology 2009;110:1041–9
- Ostad A, Kageyama N, Moy RL. Tumescent anesthesia with a lidocaine dose of 55 mg/kg is safe for liposuction. Dermatol Surg 1996;22:921–7
- Habbema L. Safety of liposuction using exclusively tumes- cent local anesthesia in 3,240 consecutive cases. Dermatol Surg 2009;35:1728–35
- Coldiron BM, Healy C, Bene NI. Office surgery incidents: what seven years of Florida data show us. Dermatol Surg 2008;34:285–91
- Grazer FM, de Jong RH. Fatal outcomes from liposuction: census survey of cosmetic surgeons. Plast Reconstr Surg 2000;105:436–46
- Lehnhardt M, Homann HH, Daigeler A, Hauser J, Palka P, Steinau HU. Major and lethal complications of liposuction: a review of 72 cases in Germany between 1998 and 2002. Plast Reconstr Surg 2008;121:396e–403e
- Moisés EC, Duarte Lde B, Cavalli Rde C, Marques MP, Lanchote VL, Duarte G, da Cunha Pharmacokinetics of lidocaine and its metabolite in peridural anesthesia administered to pregnant women with gestational diabetes mellitus. Eur J Clin Pharmacol 2008;64:1189–96
- Olkkola KT, Isohanni MH, Hamunen K, Neuvonen The effect of erythromycin and fluvoxamine on the phar- macokinetics of intravenous lidocaine. Anesth Analg 2005;100:1352–6
- Yang LQ, Yu WF, Cao YF, Gong B, Chang Q, Yang Potential inhibition of cytochrome P450 3A4 by propofol in human pri- mary hepatocytes. World J Gastroenterol 2003;9:1959–62
- Copeland SE, Ladd LA, Gu XQ, Mather LE. The effects of general anesthesia on whole body and regional pharma- cokinetics of local anesthetics at toxic doses. Anesth Analg 2008;106:1440–9
- Managing risk during transition to new ISO tubing connec- tor standards. Sentinel Event Alert. The Joint Commission. http://www.jointcommission.org/assets/1/6/SEA_53_ pdf. Accessed January 14, 2016
- Klein JA, Kassarjdian Lidocaine toxicity with tumescent lipo- suction. A case report of probable drug interactions. Dermatol Surg 1997;23:1169–74
- Martínez MA, Ballesteros S, Segura LJ, García M. Reporting a fatality during tumescent liposuction. Forensic Sci Int 2008;178:e11–6
- Rao RB, Ely SF, Hoffman RS. Deaths related to liposuction. N Engl J Med 1999;340:1471–5